 |
Hydrogen Bonding and the Anomalous Properties of Water
We are so familiar with the properties of water that it is difficult to appreciate the extent to which its behavior is unusual.
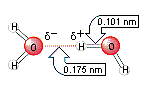
These anomalous properties all result from the strong intermolecular bonds in water. Water is best described as a polar molecule in which there is a partial separation of charge to give positive and negative poles. The force of attraction between a positively charged hydrogen atom on one water molecule and the negatively charged oxygen atom on another gives rise to an intermolecular bond, as shown in the figure below. This dipole-dipole interaction between water molecules is known as a hydrogen bond.
 |
Hydrogen bonds are separated from other examples of van der Waals forces because they are unusually strong: 10-12 kJ/mol. The hydrogen bonds in water are particularly important because of the dominant role that water plays in the chemistry of living systems. Hydrogen bonds are not limited to water, however.
Hydrogen-bond donors include substances that contain relatively polar H-X bonds, such as NH3, H2O, and HF. Hydrogen-bond acceptors include substances that have nonbonding pairs of valence electrons. The H-X bond must be polar to create the partial positive charge on the hydrogen atom that allows dipole-dipole interactions to exist. As the X atom in the H-X bond becomes less electronegative, hydrogen bonding between molecules becomes less important. Hydrogen bonding in HF, for example, is much stronger than in either H2O or HCl.
The hydrogen bonds between water molecules in ice produce the open structure shown in the figure below. When ice melts, some of these bonds are broken, and this structure collapses to form a liquid that is about 10% denser. This unusual property of water has several important consequences. The expansion of water when it freezes is responsible for the cracking of concrete, which forms potholes in streets and highways. But it also means that ice floats on top of rivers and streams. The ice that forms each winter therefore has a chance to melt during the summer.
 |
| The structure of ice. Note that the hydrogen atoms are closer to one of the oxygen atoms than the other in each of the hydrogen bonds. |
The figure below shows another consequence of the strength of the hydrogen bonds in water. There is a steady increase in boiling point in the series CH4, GeH4, SiH4, and SnH4. The boiling points of H2O and HF, however, are anomalously large because of the strong hydrogen bonds between molecules in these liquids. If this doesn't seem important, try to imagine what life would be like if water boiled at -80oC.
 |
The surface tension and viscosity of water are also related to the strength of the hydrogen bonds between water molecules. The surface tension of water is responsible for the capillary action that brings water up through the root systems of plants. It is also responsible for the efficiency with which the wax that coats the surface of leaves can protect plants from excessive loss of water through evaporation.
The unusually large heat capacity of water is also related to the strength of the hydrogen bonds between water molecules. Anything that increases the motion of water molecules, and therefore the temperature of water, must interfere with the hydrogen bonds between these molecules. The fact that it takes so much energy to disrupt these bonds means that water can store enormous amounts of thermal energy. Although the water in lakes and rivers gets warmer in the summer and cooler in the winter, the large heat capacity of water limits the range of temperatures that would otherwise threaten the life that flourishes in this environment. The heat capacity of water is also responsible for the ocean's ability to act as a thermal reservoir that moderates the swings in temperature that occur from winter to summer.